[ad_1]
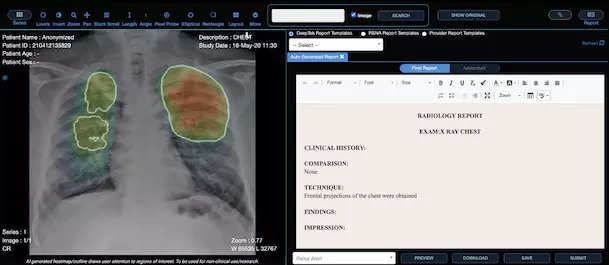
Pune: DeepTek.ai has introduced that it has acquired clearance from the US Meals and Drug Administration (FDA) for its Chest X-ray AI resolution, the CXR Analyser. The expertise makes use of deep studying algorithms to detect abnormalities in chest X-rays. It identifies, categorises, and highlights suspicious areas robotically, aiding clinicians in making exact interpretations.
The Chest X-ray AI resolution covers your complete chest space, providing evaluation for a broad vary of lung, pleural, cardiac pathologies and international our bodies/{hardware} on the chest. It is suitable with varied X-ray machines, together with hospital-based, cellular, transportable, hand-held, and ultra-portable models, making it versatile and highly effective.
The AI mannequin accelerates decision-making by detecting and finding suspicious lesions in chest X-rays, that are essential for diagnosing circumstances like lung infections, most cancers, and continual lung illnesses. In a world with 3.5 billion annual X-rays, 1.5 billion are chest X-rays, making the necessity for exact interpretation important. The scarcity of radiologists additional emphasises the function of AI in addressing this problem.
Dr. Amit Kharat, Co-Founder, DeepTek.ai, mentioned, “DeepTek CXR Analyser v1.0 is a game-changer, lowering radiologist workload by a powerful 30–50 %. With FDA clearance, we’re not solely accessing the huge US healthcare market but in addition impacting lives globally.”
[ad_2]
Source link